Qlever Software
Qlever is a software for acquisition, analysis and visualization of measured data from TMI-Orion autonomous data loggers placed at the heart of the processes.
Qlever allows you to:
- define the conditions of use of TMI-Orion and customer equipment,
- collect raw data,
- perform calculations and create business-oriented measurement or validation technical reports.
Qlever software is easy to use, intuitive, and allows customization by the user.
Definition of validation parameters and process qualification
Generation, verification and approval of business-specific reports
Optional normative compliance, depending on application modules
Two versions are available. The choice is made according to the use:
Qlever: Software platform dedicated to the management of one or several TMI-Orion data loggers.
Modules to be combined with Qlever:
- Authentication-Tracking module - FDA 21 CFR part 11,
- Pharma module - FDA 21 CFR Part 11,
- Autoclave validation module - ISO 17665/ EN 13 060/ EN 554/ EN 285/ EN 868,
- Mapping module - FDX15-140 et IEC60068.3.11,
- Module calibration -
- Washing-disinfection module - ISO 15883,
- Ceramic module.

Qlever Lite: Simplified software solution intended for managing a single wired TMI-Orion data logger.
Cannot be combined with any of the sofware modules.
Data processed and presented in a comprehensive validation and qualification report in accordance
with applicable standards (optional).
Creation of business/application recipe libraries (setup) describing the configuration, programming of TMI-Orion loggers and customer equipment, and the calculations to be made on the data collected.
Data and access traceability, track changes for compliance with FDA 21CFR Part 11 guidelines (optional).
Real-time supervision of industrial processes thanks to FullRadio or TMI-Orion radio data loggers placed at the heart of the processes.
| Module Authentification-Traçabilité - Conformité FDA 21 CFR Part 11
Gestion sécurisée des accès des utilisateurs avec création de différents comptes et niveaux d’accès (Administrateur, approbateur, opérateur). Vérification et approbation de rapports.
Traçabilité complète des processus et des données incluant toute opération d’ajout, suppression, modification (Journal d’audit).
| LDAP module for user management
In order to optimize the consistency of access control throughout the company's IT system, the administrator has the option of using Microsoft Windows® Active Directory LDAP to replace Qlever's user account management. This module requires the Authentication-Tracking module.
| Mapping module
Data treated and presented in a report, compliant with FDX15-140:2024 standard.
Intended for climatic and thermostatic chambers - or any kind of thermal regulation devices such as rooms, ovens, autoclaves - characterization and checking of temperature and humidity.
Door opening and power failure tests with recovery time calculation.
Creation of relative humidity calculated channels:
- With temperature logger placed in the chamber + a temperature and relative humidity logger,
- Or with temperature loggers placed in the chamber + a Optidew® hygrometer.
| Pharma Module - Integrates the Authentication Tracking module
Compliant with FDA 21 CFR Part 11 guidelines.
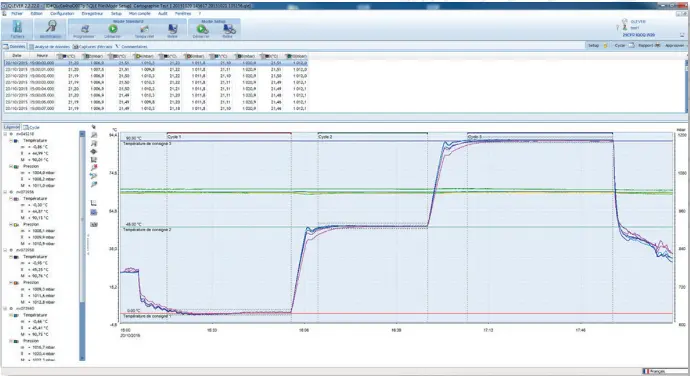
Dedicated to the analysis and operational qualification of thermal cycles in enclosures.
Output of a complete measurement report with detailed statistical calculations including up to 3 cycles and 3 steps/cycle.
| Calibration module
Dedicated to TMI-Orion temperature and humidity loggers calibration process: calibration, adjustment, checking and editing of a report.
Available with a library of drivers, to communicate with a wide variety of calibration equipment: baths, ovens, reference probes.
Delivers a calibration and adjustment report. Available with Expert mode, Automatic mode or Manual mode.
| Autoclave validation module
Data processed and presented in a comprehensive validation report in accordance with ISO 17665 / EN 13060 / EN 554 / EN 285 / EN 868.
Dedicated to autoclave qualification and to sterilization cycle validation (Bowie & Dick, Prions, Helix, ....).
Analysis of the leak test, pre-treatment phases, tray, etc.
Saturated steam calculation, F0 sterilization value calculation, dynamic pressure calculation, exhaust air calculation for heat penetration analysis in sterilization cycle pretreatments (Helix, Bowie & Dick, Prions).
| Washing-disinfection module
Data processed and presented in a comprehensive validation report in accordance with ISO 15883.
Dedicated to the qualification and validation of washing and disinfection cycles.
Analysis of the washing and disinfection phases. From 1 to 3 cycles and from 1 to 3 washing phases and 1 disinfection phase per cycle. Calculation of the A0.
| Ceramics module
Thermal profile mapping in various ponts of a cart load during the entire industrial curing cycle.
Kiln description: kiln length and number, lenght and duration of pushes.
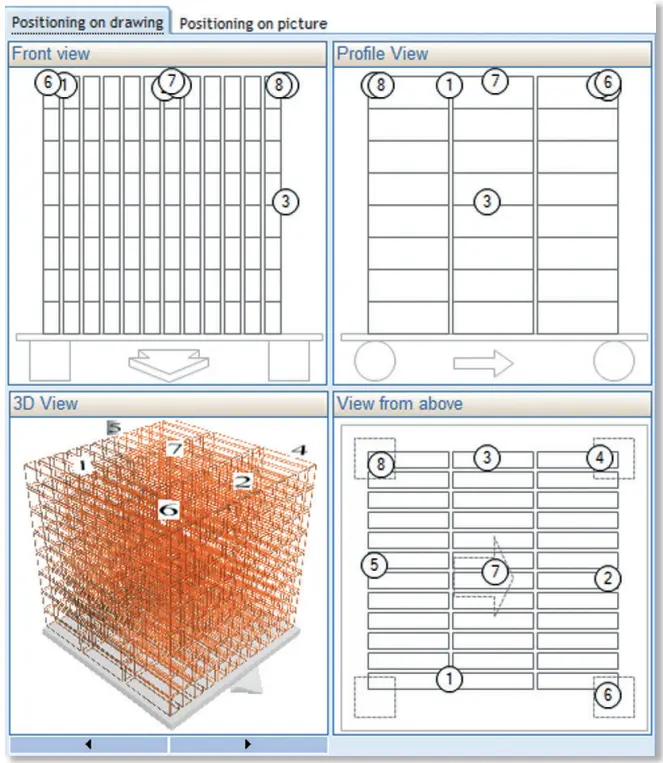
Load description: positioning and 3D visualization of thermocouple probes, types and number of products.
Kiln configuration: Position of burners, probes, fans, etc..inside the kiln.
Pushes and events management: cart thermocouple probes temperature visualization according to cart localization inside the kiln.
Report: Presentation of data in a complete measurement report.