MMS Group has acquired TMI-Orion and TMI-USA activities to complement and reinforce JRI and CIET Pharma existing offer with validation solutions designed for harsh environment
The Metrology & Monitoring Solutions - MMS Group, a global leader in metrology and temperature monitoring for pharmaceutical, healthcare, food and other regulated industries, announces the strategic acquisition of TMI-Orion and TMI-USA activities. The integration of TMI solutions will strengthen JRI and CIET Pharma's portfolio with TMI's 30+ years of expertise in reliable, user-friendly loggers and software for harsh environment validation.
Pascal Vermeersch (President of MMS) is proud to onboard Alexander Kuhnel (current CEO of TMI-Orion) and Guillaume Favre (current CEO of TMI-USA) in MMS Group along with IK Partners.
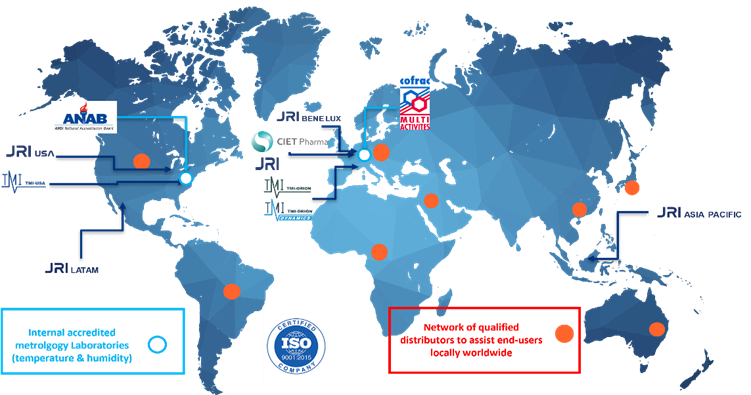
The MMS Group now employs circa 200 experts in 8 geographic areas with customers in 65 countries.
Measuring, monitoring and validation in all types of environments: JRI, TMI, and CIET Pharma are now covering the entire value chain for quality control and management.
The TMI integration creates perfect synergies across all MMS entities for product R&D and software innovation, quality procedures, metrology and validation capabilities as well as customer support worldwide. The three companies offer complementary expertise: JRI's IoT sensors and JRI-MySirius platform track temperature, humidity, CO₂, and particles in real time, complemented by TMI's validation and qualification solution in extreme environment and CIET Pharma's qualification, mapping, and compliance services.
3 companies offering a comprehensive & complementary range of services and products to meet the expectations of the most demanding customers, each with its own area of expertise with a worldwide coverage.
TMI and JRI share common DNA including metrology, quality management and customer satisfaction.
Not only do TMI and JRI share complementary product offer and R&D expertise to address similar customers, but both companies have strong expertise in metrology, data collection, transfer, storage and integrity, quality management, compliance with regulations and standards (ISO 17025, GxP, FDA 21 CFR part 11, HACCP, FD X15-140, etc.), and share common priorities in customer support and satisfaction as well as social & environmental concerns.
TMI is an expert in validation & qualification with solutions adapted to harsh environment.
TMI-Orion and TMI-USA are internationally recognized by pharmaceutical, healthcare, food and ceramic industry leaders worldwide for their high-precision embedded measurement solutions engineered for extreme environment use, serving customers such as GSK, Pfizer, Novartis, 3M, L’Oréal, Nestlé, Danone, Campbells’, Hormel Foods, Wienerberger, Imerys, Lingi, Saint-Gobain, and Airbus. Both companies are ISO 9001 certified. Their autonomous data loggers excel in ultra-high temperatures (steam sterilization, ceramics and other high-heat industrial applications with proprietary thermal shields up to 1 300°C), cryogenic lyophilization / freeze-drying (-90°C), high pressure, and ATEX zones for over 30 years.
TMI includes a USA-based operating presence with full local capabilities.
TMI-USA, based in Reston, Virginia, has an experienced team which performs the full scope TMI activities locally, from production to after sales services including customer support and metrology services with its internal metrology laboratory accredited in accordance with the recognized International Standard ISO/IEC 17025 for temperature, humidity, and pressure (with ANAB certification). TMI-USA can provide rapid response times for delivery, after sales and support to North American customers as well as LATAM customers.
TMI-Dynamics provides deep-underwater robotics equipment.
Launched in 2018 as TMI’s robotics division, TMI-Dynamics specializes in underwater solutions (Electrical Actuators, & Electrical Manipulator Arms) for critical missions of actuation, manipulation and inspection. This complementary business extends TMI's extreme environment expertise to deep-underwater equipment.
Pascal Vermeersch, President of MMS-JRI-TMI-CIET Pharma: “We are thrilled to welcome the TMI-Orion, TMI-USA, and TMI-Dynamics teams to offer our current and future customers a comprehensive range of complementary premium solutions: highly reliable, user-friendly loggers and software for validation or production processes in extreme environments. This acquisition is also a great opportunity to benefit from TMI-USA full operation in the USA including an accredited metrology lab in temperature, humidity, and pressure.”
Alexander Kuhnel, CEO of TMI-Orion & TMI-Dynamics: " Joining the MMS Group marks a historic milestone for TMI-Orion. Driven by shared values and natural synergies, this union will enable us to quickly combine our different areas of expertise to foster innovation and excellence in the services we provide to our customers. Fully aligned with the Group's ambitions, all TMI teams are committed to this customer satisfaction-focused project."
Guillaume Favre, CEO of TMI-USA: " The TMI-USA acquisition by MMS Group will empower business development actions targeted to our domestic market as well as accelerate the improvement of the datalogger product line, development of new products and features, benefiting from JRI’s technical expertise. Customer satisfaction and customer service will remain TMI-USA’s priorities."
Pierre Gallix, Partners at IK Partners: “MMS Group's acquisition of TMI enlarges MMS range of solutions and strengthens its US market expansion. Customer satisfaction and service excellence remain unwavering priorities for the Group. It also demonstrates MMS capacity to aggregate complementary and synergetic businesses to its platform.”

JRI is a metrology expert and designs, manufactures and maintains monitoring solutions for temperature, humidity, and other physical parameters adapted to the pharmaceutical industry, healthcare and food service sectors, meeting the quality requirements of the current regulations and standards (ISO 17025, GMP, GLP, FDA 21, CFR part 11, HACCP, etc.). JRI offers high-performance solutions as part of a recognized quality approach (COFRAC accreditations, HACCP, BPH, GBEA, etc.).